shelf life calculator medical device
Accelerated-aging tests are employed to generate this data for. This testing can identify film delineation and leaks.

5 X Renata Silver Oxide Watch Battery 371 Sr920sw 920 1 55v 100 Original Brand Renata 371 Renata 920 Battery Button Cell Batteries Aliexpress
Accelerated aging parameters with results supported.

. Determining a medical devices shelf life the term or period during which it remains. Return to learning center. This online service helps you to know how long your product is in good condition.
This length of time varies depending on the type of. Therefore the FDA requires medical device manufacturers to determine a products shelf life before sending the product to market in the United States. The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life.
Q10 value can be changed however default is Q1020 we recommend holding this. Accelerated aging testing is an FDA requirement for medical biomedical and pharmaceutical products. In order to calculate expiry date you should look at Production Date on your wrapping and write it.
In order to import into India Manufacturers. An accelerated ageing test for medical devices is used to simulate real time shelf-life ageing in order to validate shelf-life claims. Total period of time from production to the end of a devices life during which the device is safe and performs as intended.
A plan for the storage of shelf life samples including storage conditions. Establishing Shelf Life of Medical Devices. This process is carried out according to guidelines given in.
Type the target shelf life Days 2. Donohue J and Apostolou S Shelf-Life Prediction for Radiation-Sterilized Plastic Devices Med Dev Diag. Accelerated Aging is a process of putting packaged products into a chamber elevating the test temperature to claim a specific expiration date for a medical device product or package.
Shelf-life is the length of time you can expect a product to look and act as expected and to stay safe for use. These accelerated tests help pinpoint possible seal and burst. The shelf life of medical devices is determined by putting a device through a variety of testing procedures and many engineers follow this step by procedure to avoid any.
Materials and packaging impacts the shelf life of the device. Accelerated Aging Time Calculator. Clark GS Shelf Life of Medical Devices FDA DSMA report April 1991.
Every medical device is required to be labeled with an expiration date that is supported by shelf-life data. Type desired TAA TRT Values. It is used to simulate real shelf-life aging and is conducted to validate shelf-life claims and document expiration dates.
However sometimes it may take. Based on the type of the device Chemical Physical microbiological toxicological technological as well as packaging aspects needs to considered while determining the shelf. Shelf Life of Sterile Medical Devices.
Accelerated-aging tests are employed to generate. Sterile packaged medical devices are usually date labeled or have a pull date and may have a shelf life as defined by the 1991 FDA. The Shelf Life Rules in the importation of Medical Devices in India is of prime importance for every medical device manufacturer.
Medical Device Shelf life. Lifetime or service life. Shelf life explains duration of the medical device to be stabile to retain the sterility of the package and the device performance.

Accelerated Aging Shelf Life Testing Element

Evaluation Of Shelf Life Of Drug Products By Arrhenius Equation Part Ii Youtube

In H021 1 Medical Color Display Portable Holter Ecg Machine Price Of 3 Channel China Ecg Ecg Machine Made In China Com

Medical Device Stability Testing Guidelines I3cglobal

Stability Testing Life Science Outsourcing Inc

A Pharmacist Must Calculate The Shelf Life For An Antibiotic The Antibiotic Is Stored As A Solid And A Fresh Solution Must Be Prepared For The Patient The Antibiotic Is Unstable In

Calculating Safety Stock Sos Inventory
How To Determine The Shelf Life Of Medical Devices Previous Magazine

Simulating Packaging Barrier And Product Shelf Life Norner

Panasonic 100pcs 3v Cr2016 Cr2016 Button Coin Cell Lm2016 Br2016 Dl2016 Lithium Battery Calculator Toy Medical Device Batteries Aliexpress

Nanfu Nanfu 100 Pack Lr54 189 Coin Cell Batteries 1 5v Non Rechargeable Round Button Cell Batteries With Leak Proof Design 3 Year Shelf Life For Watches Clocks Calculator Sensor Medical Devices Toys Loblaws
.jpeg)
Panasonic Cr1632 Lithium Battery
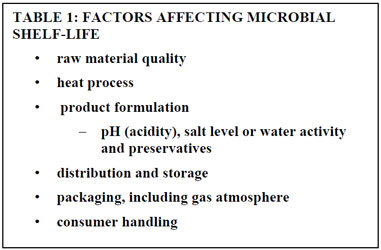
How To Determine The Shelf Life Of Food Products At Campden Bri

Medical Package Testing Lab Iso 17025 Certified Packaging Compliance Labs

Drug Stability And Stabilization Techniques Ppt Download

Calculation Of Shelf Life Of Packaging Material With Ficks First Law 1855

Accelerated Aging Testing Medical Device Accelerated Aging Westpak
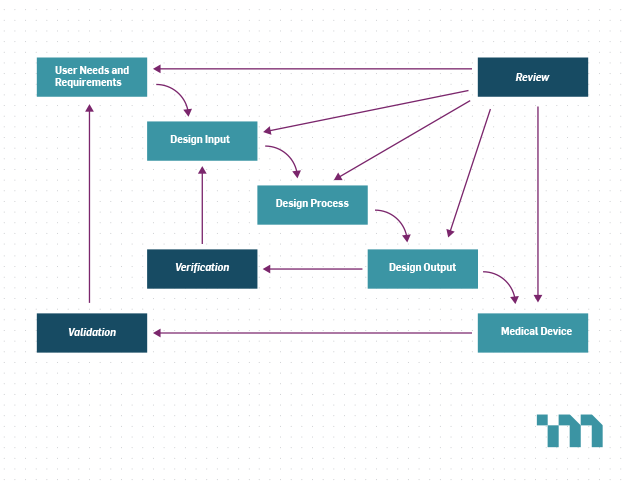
Required Verification And Validation Activities For A Fih Study